Grant of Manufacturing Licence In The Following Categories (Direct/Loan Basis/Repacking):
STEP WISE PROCEDURE
Step 1: Application for Grant of NOC from Concerned State Licensing Authority (SLA) Documents along with application: Project report and forwarding letter from DIC concerned.
Step 2: Application for Layout approval from Concerned State Licensing Authority along with two copies of original layouts which has to be certified by Concerned Drugs Inspector Manufacturing.
Step 3: Application to be submitted in the office of State Licensing Authority for grant of licence under above categories:
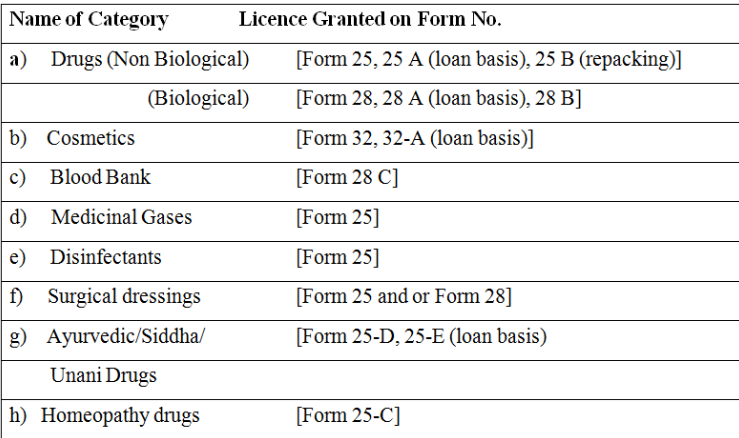
- Form 24, 24 A, 24B (Repacking), 24 C (homeopathy), 24-D (Ayurvedic and Unani Drugs), 24 E (ayurvedic Loan basis) 27, 27 A, 27-B, 27 C (Blood Bank) and 31, 31A (Cosmetics) to D&C Rules 1945. {Formats available online}.
- NOC From J&K State pollution control board for establishing a unit
- Registration of Unit with DIC, J&K State giving detail of the line of activity.
- Lease deed/ land allotment/ ownership of the land where the unit is to be established (preferably in the industrial area).
- Project Report of the Unit/Firm
- Memorandum of Article of Association/ Constitution of the unit in case of Pvt Limited.
- Partnership deed/Proprietorship (Documentary evidence with respect to Ownership deed.
- Building Plan of the unit AHU drawing.
- List of Machinery in the manufacturing Area
- List of Machinery in the QC/QA Area
- Fee applicable (Rs- 7500/- on form 24, 24 A, 27, 27 A, 24-B and 27-B (700), 27 C (for Blood Bank- Rs. 7500 + 300 for additional Blood Components) 24-C (Rs. 750/-), Rs 3500/- on form 31, 31 A and Form 24 D/24-E (Rs-1000 for Ayurvedic Licence for 5 years).
- Technical Staff- Manufacturing and Analytical Chemist as Per rule 71 and 76 of D&C Rules, 1945 along with Testimonials and Experience Certificates.
Step 4:
a) Preliminary Inspection by Concerned Drugs Inspector Manufacturing and report for Ayurvedic Licence (AYUSH) only with definite recommendation.
b) Joint Inspection from SLA and CDSCO representatives for Grant of Drugs Licence/ Cosmetic licence/Blood Bank Licence/Loan licence or repacking licence.
Step 5: Final Inspection by the SLA or Controlling Authority of Concerned State.
Step 6: Licence to be issued by SLA for all categories except Blood Bank which is to be countersigned by CLAA (Central Licensing Approving Authority i.e., DCGI)
